Smarter AI for faster, compliant research teams
Guru keeps trial info and SOPs easy to find, use, and trust—right in your workflow
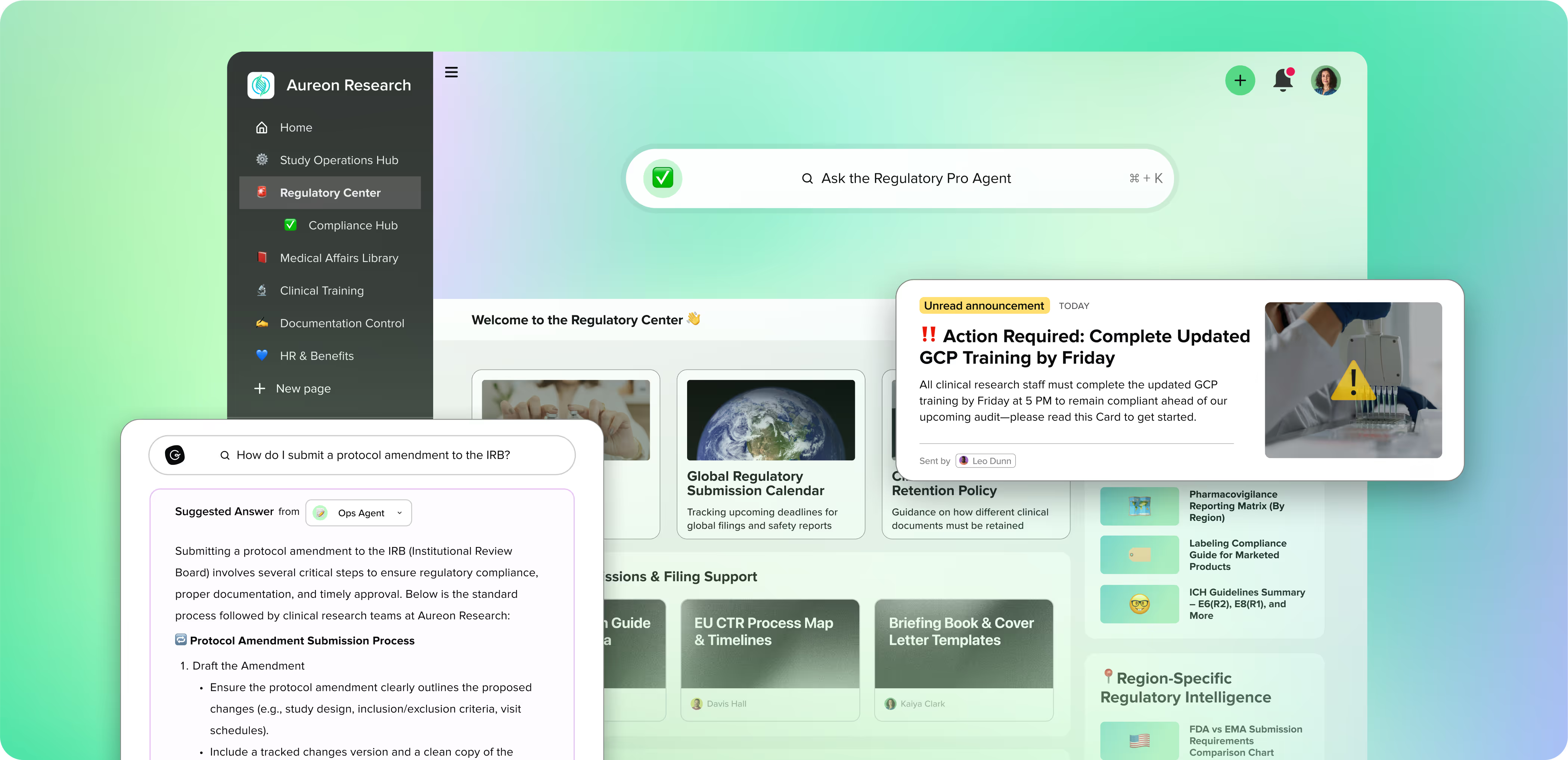







Guru keeps trial info and SOPs easy to find, use, and trust—right in your workflow








Guru surfaces trial SOPs and guidance instantly—so teams stay focused and compliant.
Guru gives MSLs and CRAs instant access to verified responses—no expert wrangling needed.
Guru centralizes FAQs and process docs so new hires ramp quickly—without overload.





























































Guru pulls SOPs, guidance, and protocols from your content—then delivers them in the tools your team already uses. Faster workflows, better compliance.








Guru’s Knowledge Agents deliver answers based on who’s asking and why—so teams get trusted, compliant info on demand.
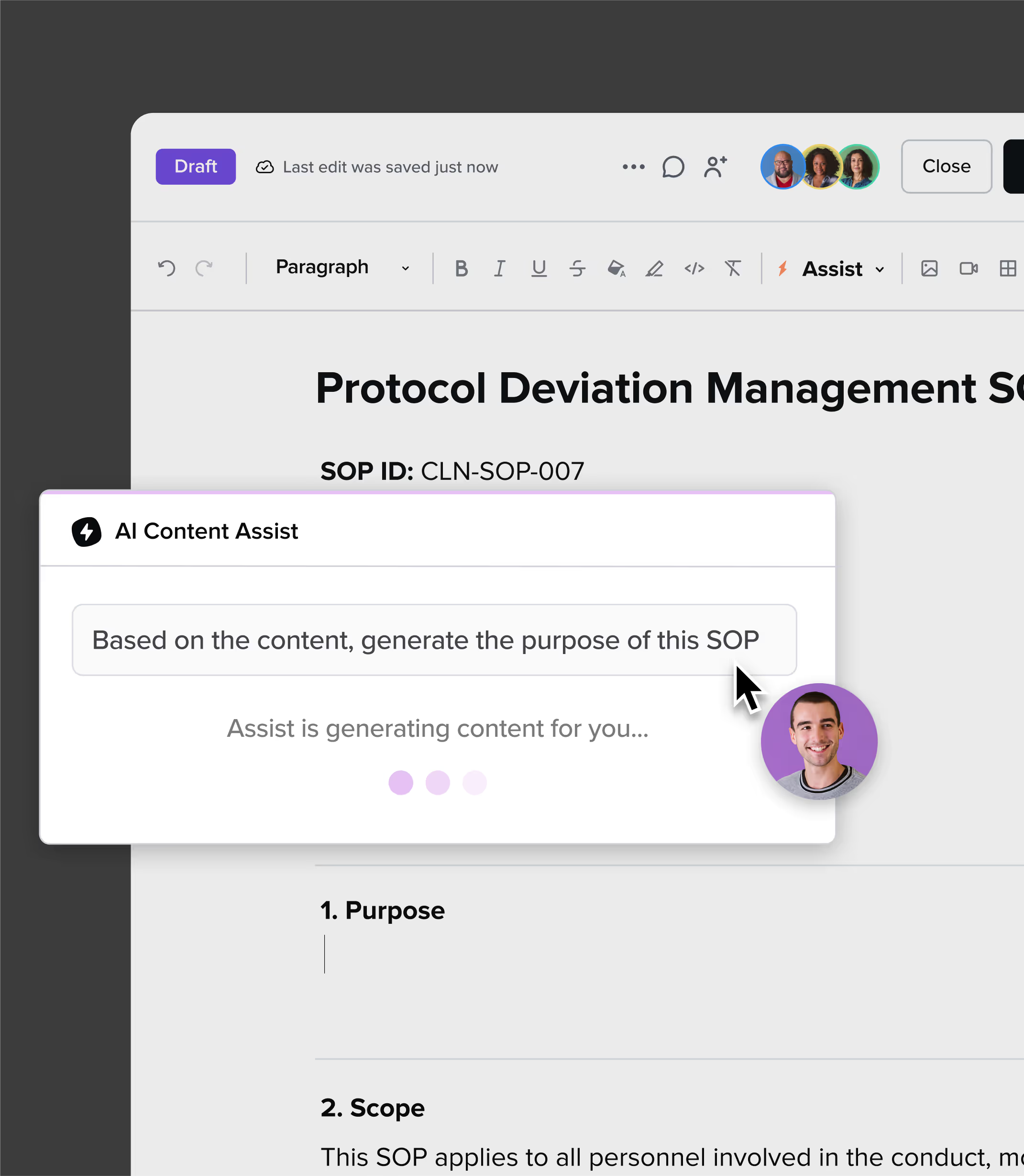
Store SOPs or link your systems—Guru makes documentation easy. AI helps refine and translate, keeping every region aligned.

Custom Pages make it easy to share protocol updates and shifts. Post once, send in chat or email, and track engagement.




Users only see (and get AI-powered answers from) content they already have access to, keeping info secure.
Allow team members to document info instantly, with expert approval before team-wide publishing.
Automatically archive outdated guidance or protocol versions to keep teams working from the latest info.
Inaccurate content risks compliance—get automatic reminders to keep critical info verified and up to date.
